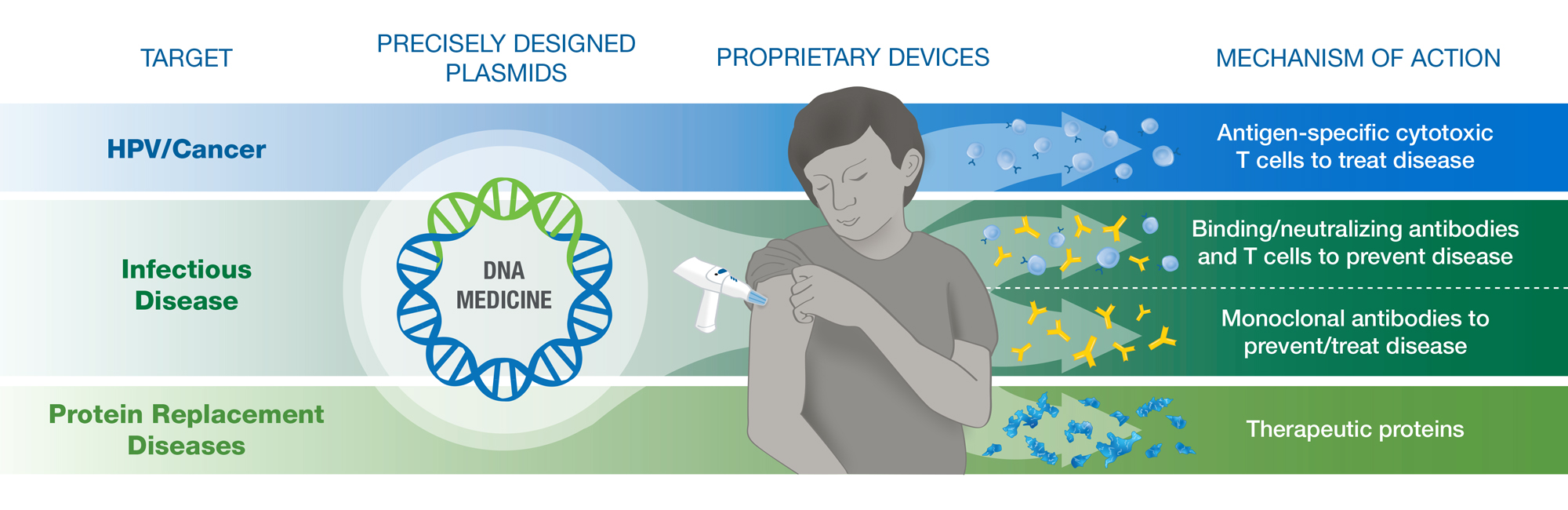
INOVIO uses proprietary technology to design DNA plasmids — small circular DNA molecules that work like software that the body’s cells can download to produce specific proteins to target and fight disease. Our proprietary investigational CELLECTRA® devices are designed to deliver the plasmids into the body’s cells for optimal effect, without the use of chemical adjuvants, lipid nanoparticles or viral vectors.

INOVIO’s SynCon® technology uses a proprietary computer algorithm to help engineer precisely designed DNA plasmids that work like software your body’s cells can download to learn how to produce a target protein. Our versatile platform can design DNA medicine candidates intended to prevent or treat a variety of disease targets.
Nucleic acid-based medicines need a pathway into the cell to work effectively. INOVIO uses proprietary delivery technology to help ensure our DNA medicines get where they need to be to make an impact – inside the cell, providing instructions to produce proteins to fight disease. After injecting the DNA medicine into either skin or muscle tissue, CELLECTRA uses electroporation (brief electrical pulses) to momentarily open small pores in the cell membrane to help the DNA plasmids enter.
We can design our plasmids to teach the body’s cells to produce a wide range of proteins, including antigens to elicit a specific immune response, monoclonal antibodies to fight a specific pathogen, or therapeutic proteins to replace defective or missing proteins in the body. Our versatile platform is able to generate antigen-targeted humoral and cellular immune responses, including antigen-specific cytotoxic or killer T cell responses that are important for fighting cancer and viral infections.
Over 19,000 doses of DNA medicines have been administered using our proprietary CELLECTRA devices to more than 6,000 patients in various clinical trials, and the data is compelling: a growing body of research indicates that DNA medicines offer many potential advantages and are generally well-tolerated, versatile, immunogenic and scalable.
© Copyright 2025 INOVIO Pharmaceuticals. All rights reserved.